SOME METHODS OF ANALYSIS AND
DIAGNOSTIS FOR CORROSION OF MATERIAL USED IN CONSTRUCTION OF VARIOUS COMPONENTS
OF NUCLEAR POWER PLANTS
abstract
In Nuclear Power Plants it is
necessary to ensure a longer and safely operating as difficult and expensive it
is the maintenance of these very complex installations and equipments. In this
regard, The Analysis and Diagnostic Laboratory Corroded Metal Components in
Nuclear Facilities-LADICON; was authorized RENAR and CNCAN notified as a
testing laboratory for nuclear-grade materials.
As part of the investigation and
evaluation of corrosion behavior for these materials are used two types of test
methods:
Longer corrosion tests such as: Autoclaving at
high temperature and pressure in different chemical media-specific patterns in
CNE and
Accelerated methods like: electrochemical
techniques accelerated chemical tests, etc.
This paper presents some methods
of analysis for materials corrosion; methods of assessment that corrosion of
structural materials exposed to specific operating conditions and environments
from CNE.
The electrochemical measurements
show the following advantages:
a) Allowing a direct method to
accelerate the corrosion processes without altering the environment,
b) It can be used as an
indestructible tool for assessing the speed of corrosion and
c) Offers the possibility of conducting such
investigations in - situ and ex- situ. Corroborating the environmental
chemistry that was born on samples movies investigation results obtained by the
methods above, it is possible to identify the types of corrosion by the material and sometimes even those
processes and mechanisms of corrosion
Introduction
Nuclear energy plants produce
electricity through the fission of uranium, not the burning of classical fuels.
Consequently, nuclear power plants do not pollute the air with nitrogen oxides,
sulfur oxides, dust or greenhouse gases like carbon dioxide (Fig.1). More than
400 nuclear power plants are operating in 25 countries around the world today,
supplying almost 17 percent of the world's electricity (Fig 2.). Many nations
are building new nuclear energy plants to meet the needs of their growing
populations and expanding economies, about 83 new nuclear energy plants being
built now around the world
During operation
of nuclear power stations (NPS) it was found that, even under normal operating
conditions of the heat transfer circuits may happen some adverse effects due in
particular to corrosion, erosion, hydrating and deposit of various corrosion
products formed on the heat transfer surfaces.
The maintenance
of a nuclear power plant (NPP) is highly complex and difficult due to its
specific nature. Thus, unscheduled stops of nuclear plants due to their
degradation by corrosion represent about 50% and in last 10 years they have
operated average about 5% lesser.
Given a set of
requirements such as:
- the increasing
of operation life in safety of structural components;
- the reducing
of the installation costs necessary to restore normal operating conditions
after stopping due to the replacement of some corroded parts;
- the reducing
of the radiation level in NPP systems;
- the avoidance
of power diminishing in nuclear power plants;
- the reducing
of the risk of losing of public confidence in those who operate nuclear
facilities,
It is necessary to know and
understand the degradation phenomena and processes of NPP structural
components.
Analysis and diagnosis of
corrosion processes leading to degradation of metal components related to
nuclear power plants and the identification of solutions to reduce/ prevent
these processes are particularly important, because the installations stopping
in view to repair or replacement of some components suppose high costs,
increases the exposure risk to radiation of operator personnel and the
radiochemical contamination risk[1].
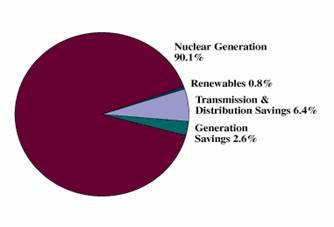
Figure 1. Contribution to CO2 Emissions
Reductions since 1973
|
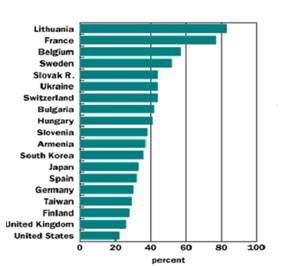
Figure 2. Percent of Electricity
Generated by Nuclear Energy (1996)
|

In the research department '
Nuclear materials and corrosion' of the SCN, there is the Analysis and
Diagnosis Laboratory of Metallic Corroded Components from Nuclear Facilities,
which is a laboratory accredited by RENAR according to ISO / IEC 17025:2005 -
"Competence general requirements in case of testing and calibration laboratories"[2].
In this paper will be presented
both some methods used in LADICON for the analysis of corroded components from
nuclear installations and testing methods used to assess the corrosion of
structural materials in similar conditions and environments with those in which
worked the corroded components.
II. Main activities of the Analysis and Diagnosis Laboratory of
Corroded Metallic Components from Nuclear Installations - LADICON
The corrosion experiments carried
in LADICON out-reactor approached the generalized and localized corrosion of
various components and structural materials using several methods and
procedures in accordance with current standards or rules, namely:
- Long period
chemical testing (static autoclavizing at those medium parameters that simulate
the operation conditions of the NPS circuits);
- accelerated
tests (using for example electrochemical methods).
II.1.
Research activity
Referring to water chemistry and corrosion problems in NPP
circuits, some research activities develop in the frame of Research
Experimental Programs referring to the corrosion of structural materials from
the primary circuit, the Steam Generator (SG) and the secondary circuit of
nuclear power plants in normal and abnormal operating conditions. These research
programs have emerged as a necessity in providing of technical and scientific
support for the safe operation and increasing the life span of nuclear power
plants from our country.
To follow the effects of water
chemistry on the corrosion and radioactivity generating by some structural
materials and to demonstrate the influence of transport activity in circuits
from Cernavoda NPS. At Cernavoda NPS there is a system of 4 autoclaves in the
'bypass' of the primary circuit. In Y1 and Y4 autoclaves existing at
the exit of the reactor is determined and studied the corrosion at 310oC,
after the outlet of heat agent from fuel channels; in Y2 and Y3 autoclaves,
placed at the inlet in reactor, is determined the corrosion at 265oC,
in operation conditions of secondary circuit after heat output from Steam
Generator. Using some devices having an adequate geometry, supplementary to
general corrosion can be studied and other corrosion types such as: stress
corrosion cracking, galvanic corrosion, crevice corrosion, corrosion of heat
affected zones (HAZ), etc.
Using both the samples tested in
autoclaves placed in primary circuit bypass and those obtained from the
decommissioned components, can execute some studies on contaminated surfaces
which follow the optimization of decontamination
and descaling procedures of surfaces covered with several deposits and can be
tested new better structural materials, in view of their adoption as
replaceable materials.
Using all these inlet data and
experimental results, was created a data basis entitled "Corrosion" that
includes all data describing the corrosion behavior of some structural
materials using the results of experiments executed out-reactor. In data basis
"CNE Corozi Test" are included the data referring to the characterization and
post-testing examinations of samples exposed in autoclaves system Y1-Y4, after
several operation periods of CANDU-6 reactor.
II.2. Analysis and diagnosis activity
Considering that the analysis and
diagnosis activity of corroded metal components from nuclear installations is
extremely important for maintaining of nuclear safety, it shall be accomplished
in conformity with the legislation of the National Commission for the Control
of Nuclear Activities -CNCAN.
Consequently, the notification of
this testing laboratory LADICON was made by CNCAN - Bucharest (notification no. Li-04-2008) for
the following analysis and evaluation methods:
. the gravimetric analysis of
corrosion coupons;
. metallographic microscopy of
corrosion coupons and respectively of parts taken from corroded metal
components in nuclear installations;
. determination of corrosion
behavior of structural materials using electrochemical methods.
It is known that most metal
components and in particular those made of carbon steel suffer some corrosion
processes in aqueous environment at high temperatures and pressures, similarly
conditions existing in primary and secondary circuits of CANDU NPP. From this
resulted the importance of execution of
corrosion tests in those conditions that simulate the best the conditions from
those circuits - primary and secondary -, in view of prognosis of the
advancement direction of corrosion and of detecting and understanding of
corrosion mechanisms.
III.1. Investigation of corrosion behavior of materials using the
autoclavizing in aqueous solutions at high temperatures and pressures
Corrosion
testing of coupons using the autoclaving exposure implies the coupons exposure
taken from different structural materials in aqueous medium or steam at high
temperatures and pressures for limited periods of time. The working parameters
and testing environment should be similar to operation conditions in which have
been corroded the samples studied.
The main equipments used in this
type of corrosion testing are[3][4]:
- one liter
Prolabo autoclave: maximum working temperature 400oC ( 5oC
the measurement error) and maximum pressure 20 MPa ( 0.5 MPa the measurement
error);
- Autoclaves
Baskerville of 5litres: working max temperature ( 5oC the
measurement error) and maximum pressure 20 MPa ( 0.5 MPa the measurement
error) (Fig. 3.)
- Autoclave
Baskerville of 13litres: working maximum temperature ( 5oC the
measurement error) and maximum pressure 20 MPa ( 0.5 MPa the measurement
error).
- A
decontamination (descalling) installation in dynamic regime;
- one liter
electrochemical autoclave which allows electrochemical measurements such as:
open circuit corrosion potential, chronoamperometrie, voltammetry, etc, at
specific parameters of nuclear power plant circuits (max.temp. 3000C,
pressure 100atm)[5]. (Fig.4.)
III.2. Application of
gravimetry at samples corrosion evaluation
Gravimetry is a quantitative
analytical method based on the mass measuring of the corrosion coupons before
and after their exposure a well-defined period of time in different
environments. The operations implied in gravimetric analysis differ from one
type of material to another. Based on initial weighing and those recorded, for
example, after autoclaving, it can determine the weight variation - ΔG -
in mg which shall be reported to the area calculated in dm2 .
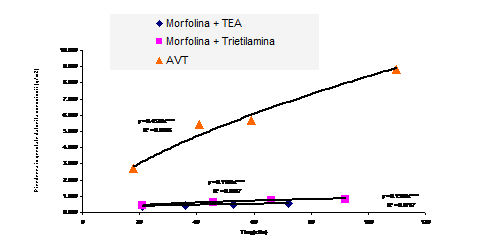
Fig.5. Weight loss function of autoclavization periods curves
corresponding to carbon steel samples autoclavized in three types of solutions
On the basis of the variation in
weight determined as ΔG/S (mg/dm2), reported at the exposure
time expressed in days, it can estimate the corrosion rate in mg/dm2 day
(Fig.5). Based on gravimetric determinations as variations of weight gains may
be made and an estimation of the thickness of superficial compounds, provided
knowledge of type compound and its density.
III. 3. Electrochemical methods
It is known that the electrochemical
parameters can furnish some data referring to the corrosion susceptibility of
structural materials used in a NPS. The main electrochemical methods are: the variation
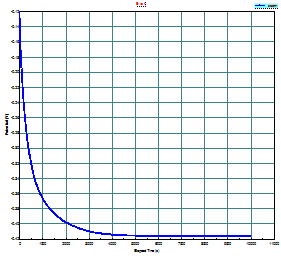
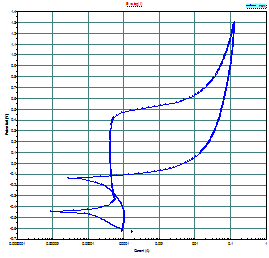
of open circuit corrosion potential function of time (Fig. 6), the linear and
cyclic polarization, the potentiodynamic, potentiostatic, galvanostatic and
electrochemical impedance spectroscopy (EIS) method (Fig.7). The last method
(EIS) implies the evaluation of the performance of superficial films deposited
on the surface of metals and has the advantage of not accelerate the
electrochemical reactions from the interface metal/ solution[8].
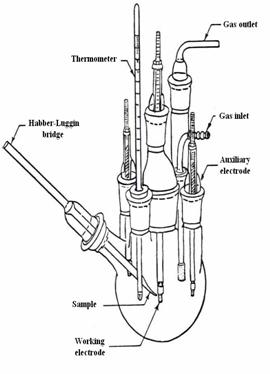
To execute the electrochemical measurements
is used a Princeton Model 2273 electrochemical system (Fig.8 and 9)
which includes: a potentiostat / galvanostat, the electrochemical cell itself
(Fig. 9) and a Windows XP computer for data processing[9].
The
electrochemical impedance spectroscopy method (EIS) is widely used in corrosion
investigation because it has the following advantages[10]:
- uses very
small signals which do not disturb the properties of electrodes;
- it can be
studied the corrosion processes and determined the corrosion rates in fluid
having very low conductivity, in which the traditional methods cannot be used;
- executing a
single measurement, can be obtained at least two electrical characteristics of
superficial films: electrical double layer capacity and polarization
resistance.
|
|
|
|
Figure
6. Evolution of potential in time
|
Figure 7. Typical curve of cyclic
polarization
|
|

|
|
|
Figure 8. Overall electrochemical potentiostat-galvanostat
PAR 2273
|
Figure 9. Sketch of an electrochemical cell
|
III.4. Metallographic analysis
Microscopic or metallographic
analysis consists in the microscopic examination of metallic materials. It is
an indispensable method in the study of materials microstructure, allowing the
determination of following parameters: the determination of microscopic
non-metallic inclusions in metals and alloys, study of modifications of the
crystalline grains, the determination of their distribution and morphology of
crystallites after size, etc.
The microscope Olympus GX 71
(Fig.10) is an optical instrument used at magnifications ranging from (x12. 5 -
x2000). The microscopic images reflect the morphology of superficial layers of
oxides and the deposition of corrosion products formed on samples. For
acquisition, processing and archiving of images, the microscope is equipped
with a working PC and software [6].
This analysis method provides
information referring to the existing grains and crystalline phases, their
distribution and nature, the crystal sizes, the morphology of hydrides, etc. It
also may reveal the morphology of corroded surface, the appearance in section
of the superficial films and could determine both the thickness and uniformity
and continuity of superficial films [7].
Conclusions
1. Activity in the LADICON is divided in two directions: research
referring to water chemistry and corrosion in NPP circuits and corrosion
behavior diagnosis and analysis of corroded metal components of nuclear installations.
2. The corrosion experiments dealing with generalized and localized
corrosion using long periods chemical tests in static autoclaves and
respectively the accelerated tests such as electrochemical tests. Evaluation of
corrosion behavior is made by gravimetric calculations and surface analysis
techniques.
3. The electrochemical impedance spectroscopy (EIS) method is used
at the study of corrosion processes and analysis, of adherence properties and
porosity of the films formed as result of corrosion[11].
4. Gravimetric analysis
allows the determination of corrosion rates, of the releasing of the corrosion
products or metallic ions during corrosion, as the total quantity of whole
formed products by corrosion, adherents and released products in corrosive
environment.
5. The metallographic
analysis is a complex interesting method consisting in macroscopic and
microscopic examination of metallic materials.
6. To investigate and evaluate the corrosion behaviors of
structural materials in view of investigate the reasons of corrosive
degradation are currently used some electrochemical methods such as: the
cyclic, potentiodynamic and linear polarization, the variation in time of open
circuit potential and the electrochemical impedance spectroscopy.
References:
[1] I.
Pirvan s.a, "Studiul cerintelor din
reglementari si descrierea metodelor de analiza si caracterizare la coroziune
generalizata si localizata a componentelor metalice corodate" R.I. 7721/
2006
[2] M.Fulger " Manualul Calitatii MC LADICON, Ed5, act 0
/2009
Li-TH-06
- Instructiuni de lucru la autoclava Baskerville de 5l;
[4] Li-TH-08 -
Instructiuni de operare pentru autoclavele Prolabo de 1l.
[5] LI-TH-203 - Operarea autoclavei
electrochimice,
[6] ASTM E
3/2007: Standard Methods of Preparation of Metallographic Specimens
[7] ASTM E
7/2003: Standard Definitions of Terms Relating to Metallographic
Scully J.R., "Corrosion Tests and Standards:
Application and Interpretation" Ed.R.Baboian (1995);
[9] "Handbook on
Corrosion Testing and Evaluation" Ed.Ailor W.H. (1971);
[10] Scully J.R., "Corrosion Tests and Standards:
Application and Interpretation" Ed.R.Baboian (1995);
[11] "Handbook on
Corrosion Testing and Evaluation" Ed.Ailor W.H. (1971);











